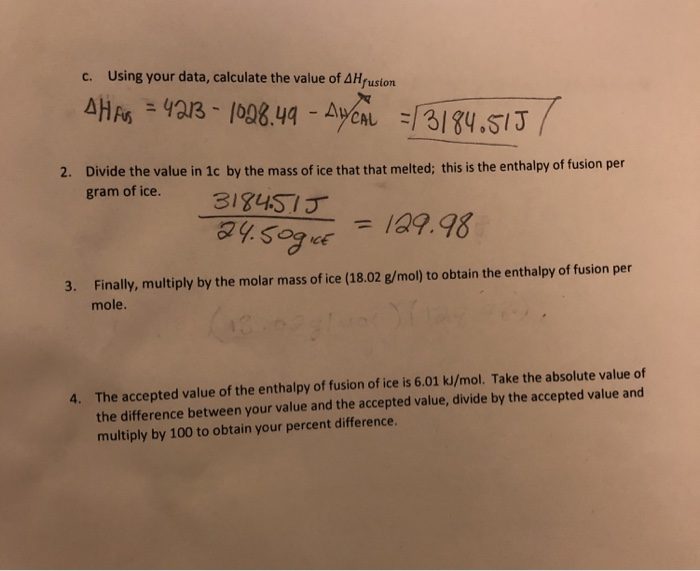Question Video: Finding the Specific Latent Heat of fusion of Water given Its Mass, the Rate of Energy Transfer, and the Time Taken to Change State | Nagwa

4 kg of ice at-20^(@)C is mixed with 10 kg of water at 20^(@)C in an insulating vessel having a negligible heat capacity. Calculate the final mass of water remaining in the

Calculate the enthalpy change on freezing of `1 mol` of water at `10^(@)C` to ice at `-10^(@)C`. - YouTube

The enthalpy of fusion of ice is 6.02kJ mol^-1 . The heat capacity of water is 4.18J(g^oC)^-1 . What is the smallest number of ice cubes at 0^oC , each containing one

OneClass: Enthalpy of a Phase Change Heat, q, is energy transferred between a system and its surround...










:max_bytes(150000):strip_icc()/GettyImages-1070123348-c9a136e91e7f4b6f9848581aa28b26d1.jpg)